Inside BENEO’s new pulse plant: pioneering sustainable protein from faba beans
New formulations provide up to 60% more protein (amino acid) than current Clinimix formulations while delivering less dextrose
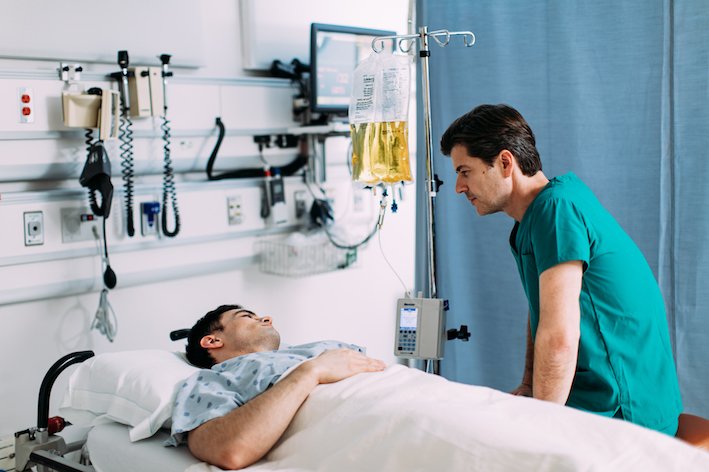
Baxter International Inc. has announced the U.S. Food and Drug Administration (FDA) approval of new formulations of Clinimix (amino acids in dextrose) Injections and Clinimix E (amino acids with electrolytes in dextrose and calcium) Injections.
These new Clinimix formulations contain up to 80 g/L of amino acids, the highest protein in any multi-chamber bag available in the U.S., making it easier to reach patient protein targets while delivering less fluid and dextrose than provided by existing formulations.
Parenteral nutrition (PN) is an intravenous administration of nutrition that plays an important role in helping reduce malnutrition and may include proteins (amino acids), carbohydrates, lipids (fats), electrolytes, vitamins and other trace elements. Guidelines from the Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN) recommend between 1.2 and 2.0 grams of protein per kilogram of body weight per day for a critically ill adult patient, and note that many patients may benefit from protein supplementation. These patients may also have other nutritional considerations, like controlling blood glucose levels and, in many cases, restricting fluid intake.
“The introduction of Clinimix and Clinimix E formulations offers the highest protein in a multi-chamber bag in the U.S., allowing clinicians more flexibility in meeting their patients’ nutritional goals,” said Heather Knight, general manager of Baxter’s U.S. hospital products business. “Clinimix and Clinimix E with Higher Protein complement Baxter’s diverse portfolio of formulations that focus on helping improve care for critically ill patients.”
In addition to these new higher protein formulations intended for patients with moderate to high protein needs, US based Baxter will continue to provide existing formulations of Clinimix and Clinimix E for patients with lower protein needs.
The approval of Clinimix and Clinimix E with Higher Protein follows the U.S. introduction of Clinolipid (20% Lipid Injectable Emulsion), the company’s proprietary olive oil-based lipid emulsion. Clinolipid, with less soybean oil and more omega-9 fatty acid than other commercially available mixed lipid emulsions, provides an additional therapy option for use in PN for adults.